Overview
At Vancouver General Hospital’s Cytogenomics Laboratory, I directed the clinical validation, implementation, and launch of Optical Genome Mapping (OGM) as a first-line diagnostic test for hematological malignancies. This initiative, completed in November 2023, represents the first clinical deployment of OGM in Canada for acute leukemia testing and has since become an integral part of routine diagnostics at VGH.
Validation and clinical impact
- Clinical roll-out of OGM as a front-line diagnostic tool for acute leukemias.
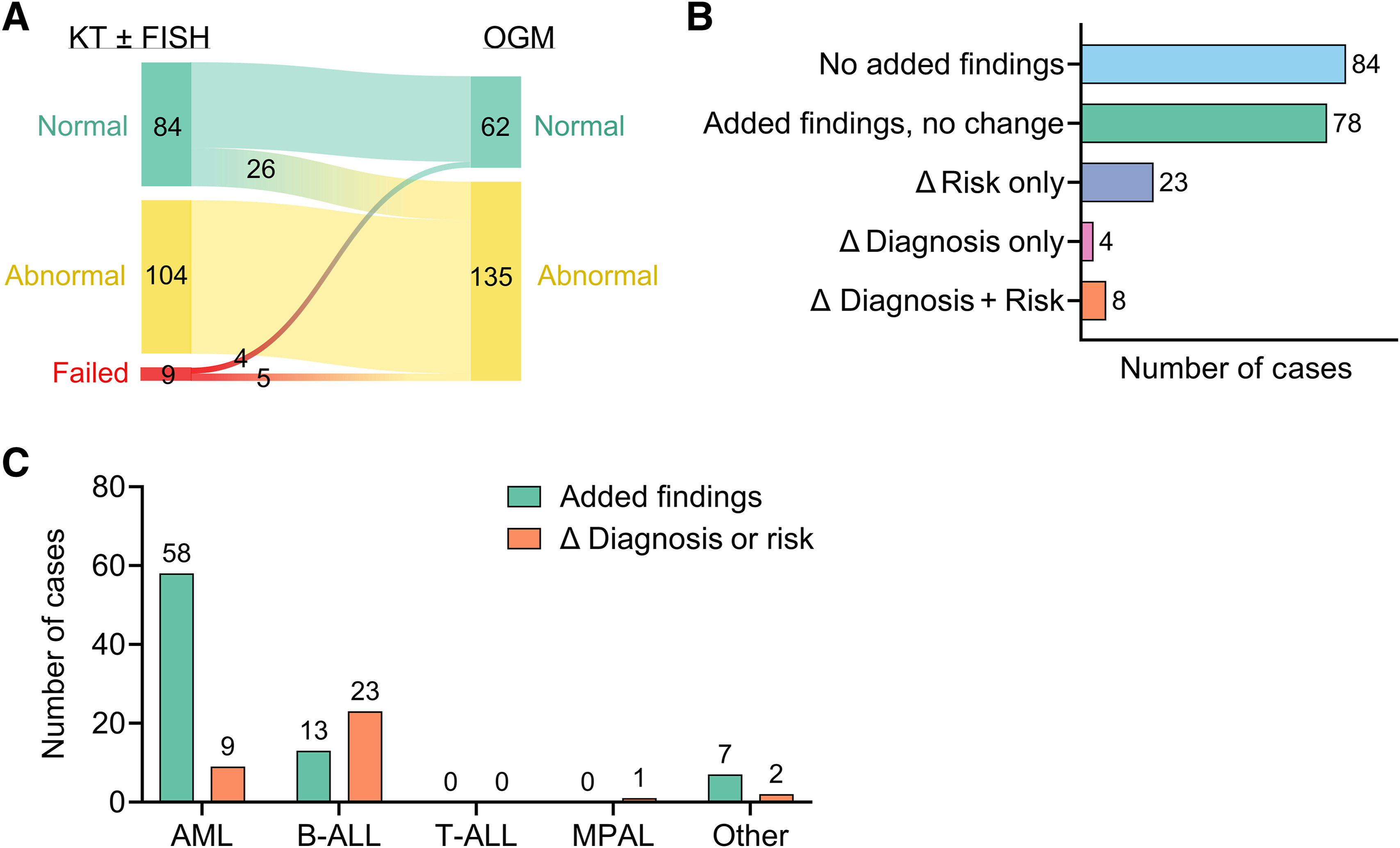
- Since implementation, the lab has profiled hundreds of patients with a 97% quality control success rate and clinically significant findings in 49% of cases—directly influencing diagnostic and treatment decisions.
- Presented findings demonstrating that OGM consistently detects clinically actionable structural variants missed by conventional cytogenetic methods.
Dissemination and recognition
The project’s validation and clinical impact has been published and widely disseminated through national and international platforms, establishing VGH as a Canadian leader in genomic innovation. It was also featured in the 2023 UBC Department of Pathology & Laboratory Medicine Annual Report, highlighting its role in advancing genomic diagnostics in British Columbia.
Publications and ongoing work
- This work is now published as "Technical Validation and Prospective Clinical Utility of Optical Genome Mapping in Acute Leukemia Workup" in the Journal of Molecular Diagnostics.
- Expanding testing to include myelodysplastic syndromes, relapsed acute leukemias, and chronic lymphocytic leukemia.
- Integrating OGM with Oxford Nanopore long-read sequencing for comprehensive structural variant characterization.
Impact and significance
This project has established a new clinical standard for detecting complex structural variants in hematologic malignancies. OGM now serves as a routine first-line diagnostic at VGH, streamlining turnaround times and providing higher-resolution insights than traditional cytogenetic testing. Ongoing efforts continue to expand the technology’s scope and demonstrate its potential as a cornerstone of precision oncology in Canada.